Abstract
Background: Vaso-occlusive painful crises (VOC) are a hallmark of sickle cell disease (SCD). Historically, efficacy of drugs intended to reduce frequency of VOCs has been evaluated solely on the frequency of VOC that require a medical intervention, such as a visit to the emergency department, an infusion center and/or hospitalization. Several studies (PiSCES, ELIPSIS) have now demonstrated that the majority of painful events are managed in the home setting.1,2 Therefore, accepted measures to evaluate disease burden do not represent the totality of the patient VOC experience. There is a need to improve and expand on existing clinical trial endpoints to capture the totality of the VOC experience. Moreover, participation in clinical trials for patients with SCD faces numerous barriers, such as transportation difficulties, burden to patient's family/work time and recently, concerns about Covid-19 exposure. We aim to quantify daily pain episodes and provide additional evidence supporting validation of responsiveness in a therapeutic setting for a novel SCD electronic patient reported outcomes (ePRO), that may be utilized in a decentralized or traditional study setting. To successfully assess this tool, it is essential that participants comply with the completion of this daily diary.
Methods: PROGRESS (NCT05407805) is a prospective study characterizing VOCs in 2 cohorts of patients with HbS/S or S/B0 Thal ≥18 years of age. Group 1, "control" consists of up to 100 patients not on disease modifiers, and group 2," Hydroxyurea" (HU) includes 50 patients on a stable dose of HU. On Day 1, patients have a blood draw for biomarker evaluation and receive training for their SCD diary on an ePRO device. Patient's participation is approximately 7 months. The blood draw and the training on the SCD ePRO device may be performed remotely by a phlebotomist in the patients' home, or at a site. The SCD ePRO diary consists of up to 6 questions and is completed in the evening to reflect the past 24 hours. Strategies to assure the diary is completed include simplification of questions and responses, device alarms, allowance to complete until noon the following day, morning calls to patients if a diary has not been completed the night before and remuneration for the completion of the diary each day. A small bonus payment is provided when 6 or more diaries are completed over a 7-day period, with the bonus increasing during the final 3 months of the study.
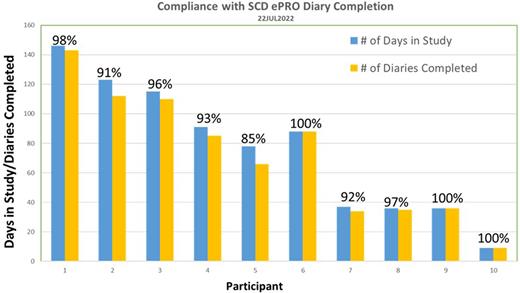
Results: To date, two sites have screened 15 individuals and enrolled 10 participants. Participants are generally able to complete the diary in approximately 1-2 minutes and the daily diary completion compliance is ~95% over an average of 75 days (range: 9 to 146 days).
Discussion: Classical methods for measuring the rate of VOCs, based solely on utilization of medical resources, vastly underestimate the pain burden in SCD; therefore, developing new validated endpoints that measure the totality of pain episodes, including home VOCs is a priority. Demonstration of sustained compliance and responsiveness of a daily diary may provide additional confidence in the use of this tool as an endpoint in broader therapeutic settings.
References 1. Smith WR, Bovbjerg VE, Penberthy LT, et al. Understanding pain and improving management of sickle cell disease: The PiSCES study. J Natl Med Assoc 2005;97(2):183-93.
2. Pittman DD, Hines PC, Beidler D, et al. Evaluation of longitudinal pain study in sickle cell disease (ELIPSIS) by patient-reported outcomes, actigraphy, and biomarkers. Blood 2021; 137(15):2010-20.
Disclosures
Minniti:Pfizer Inc: Research Funding. Bronte-Hall:Pfizer Inc: Research Funding. Marraffino:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Healy:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Rybin:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Beidler:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Huang:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Shelton:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Taylor:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Goossens:Pfizer Inc: Research Funding. Arkin:Pfizer: Current Employment, Current equity holder in publicly-traded company, Other: S Arkin's spouse is an employee of Pfizer Inc and is a current equity holder in the company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal